Three people dead after using eye drops contaminated with rare bacterial superbug
03/27/2023 / By Zoey Sky

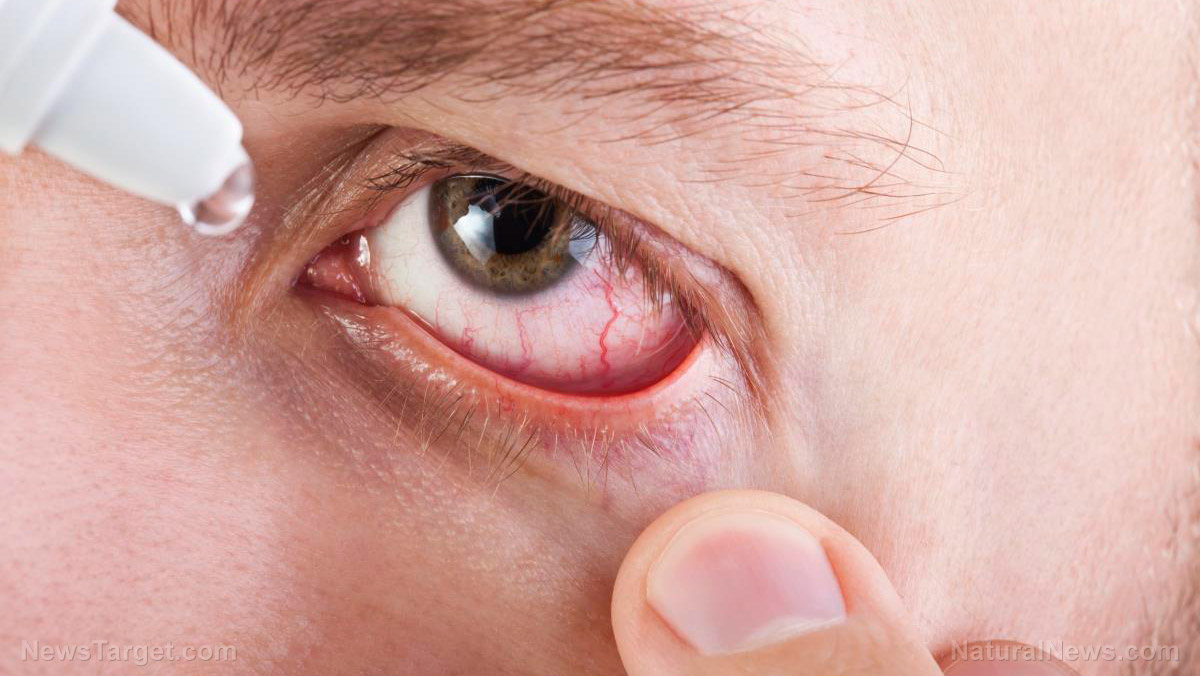
When your eyes are dry and irritated, it’s normal to use artificial tears to relieve any discomfort.
But for some unfortunate individuals, using eyedrops contaminated with a rare bacteria resulted in three deaths and eight cases of vision loss. The Artificial Tears eye drops were bought at pharmacies all over the United States.
As of March 14, 68 individuals from 16 states have been infected with a rare strain of Pseudomonas aeruginosa, reported the Centers for Disease Control and Prevention (CDC).
The CDC also announced that four of the affected individuals had to have their eyeballs removed.
Eyedrops recalled in February
Global Pharma Healthcare recalled its Artificial Tears Lubricant Eye Drops, which were distributed by EzriCare and Delsam Pharma, in February. The eye drops are often used by people with dry eyes who need extra lubrication to relieve discomfort. (Related: Clorox recalls Pine-Sol products due to bacterial contamination.)
Artificial Tears Lubricant Eye Drops are sold at drug stores such as CVS, Target and Walmart all over the country. The product is also sold by online retailers like Amazon, but they have since been recalled.
Health authorities are continuing to track infections as they investigate the outbreak in these 16 states:
- California
- Colorado
- Connecticut
- Florida
- Illinois
- North Carolina
- New Jersey
- New Mexico
- New York
- Nevada
- Pennsylvania
- South Dakota
- Texas
- Utah
- Washington
- Wisconsin
The CDC warned those who have used the eye drops, especially if they have noticed symptoms of an eye infection, to seek medical care as soon as possible.
Per the CDC, signs of an eye infection include:
- Eye pain or discomfort
- Blurry vision
- Clear, green, or yellow discharge from the eye
- Redness of the eye or eyelid
- A feeling of something in your eye
- Increased sensitivity to light
Most of the cases have been linked to four regional clusters. Data showed that Ezricare’s eye drops were the only product used by the affected individuals in those specific groups.
Most reported using at least 10 different brands of artificial tears, but EzriCare Artificial Tears, which is a preservative-free, over-the-counter (OTC) product packaged in multi-dose bottles, was the brand most commonly reported.
The recalled eye drops were manufactured by Global Pharma Healthcare in India, where P. aeruginosa is often associated with hospital outbreaks.
P. aeruginosa can spread through contaminated hands or medical equipment. Experts warned that the outbreak is alarming because the bacteria that causes it is resistant to standard antibiotics.
Pseudomonas aeruginosa and eye health
Two case studies published in the journal JAMA Ophthalmology contained more details about the infections.
In the first case, a 72-year-old woman lost vision in her left eye. She reported that she had been using EzriCare artificial tears for about a week.
Dr. Ahmed Omar, an ophthalmologist at University Hospitals Cleveland Medical Center who treated the patient, said that the patient first noticed some blurry vision in her left eye for several days.
The blurry vision was “initially painless,” but the patient woke up one morning with a yellow discharge on her pillow. It was at this time that she started noticing how the appearance of her eye had changed.
After the woman went to the emergency room, the doctors discovered a large ulcer on her left cornea. The patient was hospitalized for three weeks.
She was given IV antibiotics and antibiotic eye drops while also undergoing several surgical interventions. Unfortunately, the woman lost vision in her left eye because of serous choroidal detachment or an abnormal accumulation of fluid.
In the second case study, researchers wrote about a 72-year-old man who developed significant vision loss from an infection of the cornea.
While the man’s condition later improved, he still has vision issues. The male patient had not experienced previous eye problems.
However, after using EzriCare artificial tears for eye dryness, he experienced severe pain and went to the Bascom Palmer Eye Institute in Miami. Doctors found that he had multidrug-resistant Pseudomonas aeruginosa keratitis.
Dr. Marissa Shoji, one of the doctors who treated the male patient, reported that they discovered severe corneal infection in his right eye. The man “could only see shadows and was not able to see letters due to the extent of the ulcer.”
His doctors gave the patient strong antibiotics, but his condition worsened. Shoji explained that while the medications usually helped improve the condition of patients, when they saw him two days later, his condition had gotten “far worse.”
After this event, the doctors inquired specifically about the EzriCare tears. They knew the product was linked to resistant infection that may not respond to the strong antibiotics.
The doctors also reported that cultures from the man’s cornea and EzriCare bottle grew the same strain of multidrug-resistant P. aeruginosa.
During the man’s two-month follow-up, his vision was 20/400. This meant he can see at 20 feet what healthy people can see from at least 400 feet.
Dr. Guillermo Amescua, an ophthalmologist at Bascom Palmer Eye Institute, said that at one point, the old man “was in danger of having permanent vision loss.” Despite his recovery, the patient now has corneal blindness.
In January, after finding out about the CDC’s investigation of Pseudomonas infections, EzriCare released a statement that “immediately took action to stop any further distribution or sale of EzriCare Artificial Tears.” The company also contacted customers to “advise them against the continued use of the product.”
Tips for preventing Pseudomonas aeruginosa infections
Aside from avoiding the contaminated Artificial Tears Lubricant Eye Drops distributed by EzriCare and Delsam Pharma, you can follow the tips below to prevent P. aeruginosa infections:
- Wash your hands often and thoroughly. Proper hand hygiene is the best way to prevent the spread of germs. Use soap and running water and scrub for at least 20 seconds. If you don’t have soap and water, use an alcohol-based hand sanitizer.
- Remind others to wash their hands properly. If you’re in the hospital, ask doctors, nurses and visitors to wash their hands before touching you.
- Clean all surfaces in your home. This means disinfecting all of the surfaces you touch frequently throughout the day, like tables, door knobs, light switches and your cell phone.
- Keep your wounds clean. If you have a cut or scrape, clean it thoroughly and cover it with a bandage.
- Do not share personal items. Don’t let other people use personal items like your towel or razor.
- Swim safely. If you are going into a hot tub or pool, make sure it is properly chlorinated and maintained.
Visit Products.news to read about other products that have been recalled because of contamination.
Watch the video below to learn how to wash your hands properly.
This video is from the Daily Videos channel on Brighteon.com.
More related stories:
Laser technology used in self-driving cars can damage cameras and human eyes.
Sources include:
Submit a correction >>
Tagged Under:
artificial tears, bacterial infections, contamination, dangerous, eye drops, eye health, eye infections, EzriCare, Global Pharma Healthcare, outbreak, Product recall, product safety, Pseudomonas aeruginosa, superbugs, visual impairment
This article may contain statements that reflect the opinion of the author
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 EYEHEALTH.NEWS